Irresponsible use of antimicrobials, including antibiotics, is accelerating the rise of ‘superbugs’. These superbugs are causing infections that were once easily treatable by antimicrobials but are now becoming increasingly difficult to treat and, in some cases, turning life-threatening. Unnecessary use of antimicrobials coupled with poor infection prevention measures and lack of new antimicrobials has resulted in a full-blown global health crisis of Antimicrobial Resistance (AMR). The climate crisis is also affecting patterns of infectious diseases which may lead to an increase in the use of antimicrobial drugs and a rise in AMR.
While the problem of AMR has reached a tipping point in India, there are countless and yet, often unheard of superheroes – in research labs, hospitals, and on the ground, among communities – who are working very hard to stop the rise of these deadly superbugs. In our SaS-AMR Champions Series, commemorating the World Antimicrobial Awareness Week (WAAW2021), we bring to you conversations with some of these ‘Superheroes Against Superbugs’ that we hope would inform, inspire, and encourage us all to act against AMR to ensure a healthy future for all.
The development of new antimicrobials and other and biomedical interventions alone will not be enough to fight AMR, controlling infections in healthcare settings will also be key. Uma Bala Pamidimukkala plays the role of a Superbugs Inspector in a city hospital. She is an Additional Professor in the Department of Microbiology at Nizam’s Institute of Medical Sciences (NIMS), Hyderabad, where her work involves the identification of disease-causing microorganisms and providing information to doctors on whether or not these pathogens are susceptible to antimicrobials. This critical information in turn helps doctors to optimise treatment and in avoiding antimicrobial use when not needed. Uma Bala is also a part of the Antimicrobial Stewardship Programme that organizes different workshops to spread awareness on the different aspects of infection, disease control and its prevention to ensure infections, including AMR infections, do not escape into the environment. In this interview, Uma Bala shares her experience of working in public hospitals that are proven to be hubs for HAIs and AMR infections and talks about the various challenges that one faces in stopping the spread of AMR in healthcare settings.
Can you tell us about the role clinical microbiologists play in containing the spread of AMR infections in healthcare settings?
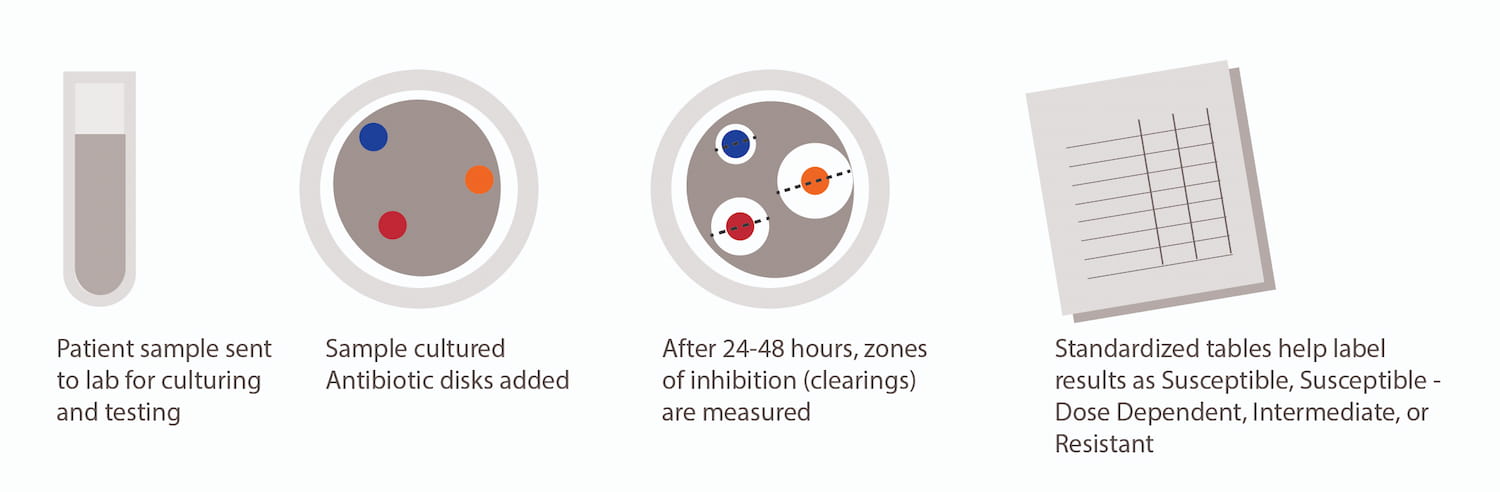
Uma: The role of a clinical microbiologist in containing the spread of AMR in health care settings is multifaceted. It involves implementing diagnostic stewardship for optimal diagnosis and management of patients with infectious diseases. They also have to oversee accurate, reliable and timely identification and antimicrobial susceptibility testing of the common pathogens causing community-acquired and Hospital Acquired Infections (HAI). Clinical microbiologists have to ensure rapid identification of epidemiologically significant antimicrobial-resistant pathogens and the underlying genetic mechanisms by molecular or proteomics-based methods. They have to apply microbiological and clinical knowledge to interpret the laboratory results and decide the clinical significance of the culture results, thereby promoting the use of antimicrobials only when required.
Clinical microbiologists are also key members of the Hospital Infection Control and Prevention Committee. They play a critical role in educating clinicians, nurses and other health care workers about infection prevention and control measures. This includes hand hygiene, standard and transmission-based precautions (contact, droplet and air-borne), disinfection and sterilization protocols, bundles for prevention of device-related and surgical site infections, surveillance of HAIs and healthcare worker compliance with infection control measures.
On the Antimicrobial Stewardship Programme (AMSP) front, clinical microbiologists have to ensure implementation of cascade reporting whereby results of Antimicrobial Sensitivity Test for broad-spectrum and expensive antimicrobials are withheld when organisms are susceptible to narrow-spectrum drugs. They are also expected to share updated information with the clinicians regarding the trends in AMR of the common pathogens in the hospital by regular release of cumulative antibiograms to assist in the appropriate choice of empiric therapy. Finally, they play a central role in developing the hospital antibiotic policy in close association with the clinicians and pharmacologists.
What are Nosocomial infections?
Uma: Nosocomial infections, also referred to as Healthcare-associated infections (HAI), are infections acquired during the process of receiving healthcare in a wide variety of settings. By definition, they are not present or incubating at the time of admission of the patient and may also manifest after discharge from the hospital.
Are these hospital-acquired infections generally resistant to commonly used antimicrobials? If so, why?
Uma: In many cases, this is true. For instance, antimicrobial-resistant pathogens such as Methicillin-resistant Staphylococcus aureus (MRSA), Vancomycin-resistant Enterococcus (VRE) and MDR Gram-negative bacteria are more prevalent in a healthcare setting.
This is largely due to excessive and often inappropriate use of multiple and broad-spectrum antimicrobials in the hospital that selects out the antimicrobial-resistant micro-organisms while killing the disease-causing ones susceptible to antimicrobials.
These pathogens are also easily transmitted to the hospitalized patient from other patients, hospital staff, or the hospital facility through the hands, contaminated instruments or the patient care environment. Finally, the underlying debilitating diseases and immune-compromised status of the hospitalized patient along with the multiple interventions, procedures or surgeries the patient is subjected to, increases the risk of acquiring these infections.
What are some protocols followed after coming across a patient harbouring a drug-resistant pathogen? And what steps are taken by the antimicrobial surveillance team to prevent the further spread of this pathogen?
Uma: Various protocols are followed starting with contact isolation precautions for patients infected or colonized with drug-resistant pathogens till the patient’s cultures are negative. Additionally, the following steps and protocols are usually taken to prevent further spread of resistant or other pathogens:
- Strict compliance with hand hygiene
- Use of appropriate PPE after risk assessment
- Implementation of prevention bundles for common HAIs such as Central line associated bloodstream infection (CLABSI), Catheter-associated Urinary tract infections (CAUTI)
- enhanced cleaning and disinfection of the patient care equipment and environment
- Use of single-use items for each patient where feasible (stethoscope, thermometer, BP instrument, bedpans)
- Grouping together patients with the same infectious disease and hospital staff looking after them to reduce opportunities for cross transmission
- Alert codes to easily identify patients
- Proper management of biomedical waste and laundry so as not to contaminate the environment
- Limiting transport of the patient in the hospital
- Limiting visitors to the patient suffering from an infectious disease
- Terminal cleaning of the patient care areas after discharge
- Active screening cultures for early identification and isolation of colonized or infected patients so that measures for isolation and decolonization are instituted
- When a common source is suspected, healthcare worker screening and environmental screening for identification of the source to prevent further spread
- Informing and educating healthcare workers about infection control measures
- Implementation of Antimicrobial Stewardship Programme
With your experience of working in a public hospital in India, what are some of the challenges faced in implementing various measures in containing AMR? What, in your opinion, is the way forward?
There are multiple challenges in addressing the problem of AMR in public hospitals in India.
Uma: To begin with, there is usually inadequate laboratory support for the diagnosis and management of infectious diseases; most microbiology laboratories in public hospitals do not have advanced equipment or sufficient, trained personnel for timely and accurate diagnosis of infectious diseases.
There is also poor implementation of infection control and prevention programmes due to the following reasons:
- No dedicated personnel like full-time Infection Control Nurses (ICNs)
- Trained housekeeping staff are not available for day to day implementation of the programme resulting in unchecked transmission of drug-resistant pathogens.
- Inadequate hospital infrastructure and inadequate supplies of PPE, disinfectants and other material for Infection Control and Prevention (IC&P) .
- No dedicated funds for the IC&P
- Lack of awareness regarding the importance of the IC&P programme among the Healthcare Workers including the administrators, doctors and nurses.
Additionally, antimicrobial stewardship is non-existent in our hospitals because of which there is irrational use of antimicrobials and there are usually no hospital antibiotic usage policies and guidelines. Local AMR data in the form of Cumulative Antibiograms in most public health hospitals are unavailable and neither an infectious disease physician nor a clinical pharmacologist is usually available to guide antimicrobial therapy.
Therefore, these would be my suggestions to improve infection control measures at public hospitals:
- Improve clinical microbiology laboratories with advanced equipment and trained personnel for effective implementation of diagnostic stewardship
- Dedicate resources and trained personnel for effective implementation of Infection Control Programme and Antimicrobial Stewardship Programme. This would involve determining requirements for personnel and infection control supplies on the basis of the hospital bed strength and level of care (for e.g., one full-time Infection Control Nurse for every 100 beds in a tertiary care hospital). Basically, minimum requirements of personnel and resources should be set for all healthcare facilities.
- Continuous monitoring and reporting of hospital infection rates, AMR as well as compliance to infection control measures to a regulatory body
- Continued training in IC&P and Antimicrobial Stewardship for doctors and nurses starting from undergraduate level
- Increasing the availability of ID specialists and clinical pharmacologists to guide antimicrobial therapy decisions
- Implementing restricted dispensing of targeted antimicrobials (formulary restriction) and preauthorization to control irrational use of broad spectrum antimicrobial use
Nizam’s Institute of Medical Sciences (NIMS) is one of the regional centres in the AMR Surveillance and Research Initiative network established by the Indian Council of Medical Research (ICMR). Can you please tell us about the work of this network and the role played by NIMS?
Uma: ICMR initiated the Antimicrobial Resistance Surveillance & Research Network (ICMR-AMRSN) in 2013 with an aim to understand the extent and pattern of AMR in India and to use this evidence to guide strategies to control the spread of AMR. The main goals of ICMR-AMRSN are to:
- establish network of hospitals to monitor trends in the antimicrobial susceptibility profile of clinically important bacteria and fungi limited to human health
- include comprehensive molecular studies for identifying the clonality of drug-resistant pathogens and their transmission dynamics to enable a better understanding of AMR in the Indian context and develop suitable interventions
- disseminate information on AMR in pathogenic organisms to stakeholders to promote interventions that reduce AMR
- create data management system for data collection and analysis
NIMS is one of the 16 regional centres that are part of the AMRS Network that coordinates data collection on AMR infections, sharing clinical isolates of the resistant pathogens with respective nodal centres and helping with integrated management, analysis and reporting of surveillance findings from this part of the country.
Since the patterns of antimicrobial-resistant pathogens change from region to region, information on the current patterns of resistance is crucial for targeted antibiotic policy.
As a microbiologist, what according to you are some key actions that medical professionals can undertake to minimize the occurrence of AMR?
- Arrive at an accurate diagnosis of an infectious disease in close coordination with the clinical Microbiology department, for optimal management of patients.
- Use antimicrobial susceptibility results to guide therapy where possible.
- Know your local AMR data to make decisions for evidence-informed antimicrobial use.
- Be aware of and strictly follow principles of IC&P and AMSP to prevent the spread of AMR.
This World Antimicrobial Awareness Week, what would be your AMR call to action to the general public in India?
- Do not take antibiotics when not required such as for the treatment of viral infections like the common cold.
- Do not self-medicate. Use antibiotics only when prescribed by a qualified medical professional.
- Strictly follow your doctor’s advice regarding the dose and duration of antimicrobials
Interview by Team SaS and Moneca Kaul; Moneca is a PhD researcher based at CCMB, Hyderabad. She is studying the bacterial cell wall and proteins that break this wall to accommodate new cell wall material as the bacterial cells grow and divides.
Cover Art by Mrunal Kulkarni
How can we solve the antibiotic resistance crisis?