Superbugs–microbes like bacteria, fungi, virus, or any parasite resistant to all commercially available antimicrobials–are on the rise, while the number of new and promising drugs in the pipeline to tackle them are underwhelming.
Bacteria are some of the most common superbugs, thanks to their unique adaptations like rapid evolution and horizontal gene transfer. A report by the World Health Organization (WHO) noted that the number of deaths due to antibiotic-resistant bacterial infections is higher than those caused by either HIV/AIDS or malaria.
We now live in the ‘post-antibiotic’ era, where many first-line antimicrobials are no longer effective in treating everyone. A key site for emerging microbial resistance are hospitals. Superbugs continue to emerge in hospital settings where they are constantly exposed to antimicrobials. The overuse of antibiotics in society, agriculture, and livestock management cripples the ability of our health systems to tackle AMR emergence. This is because such constant and uncontrolled exposure of bacteria to low dose antimicrobials (as happens in sewage water or in agricultural fields) imposes a selective pressure on them. Resistant strains of bacteria survive and multiply, and previously non-resistant strains, by a unique ability in bacteria to swap genes, gain and start expressing resistance genes.
Take Colistin, the last line of defence in hospitals against infections caused by Gram-negative bacteria like Escherichia coli. Colistin has been the reserve antibiotic for infections that are resistant to a large number of antibiotics, especially the higher antibiotic carbapenem. Today, there is growing resistance to this reserve drug as well.
“Hospitals need these miracle drugs to stave off infection and save lives. It is necessary then to understand what hospitals do to ensure the judicious use of antibiotics.”
Stewardship and Susceptibility Profiling
Many hospitals run stewardship programs that run successful interventions to improve antibiotic use linked outcomes in patients. A stewardship program balances the drug, the dose, the duration and medium (oral or topical), with resistance development and toxicity. Hospitals also run microbiology tests to sample various sources for the presence of AMR in the cultures at various sections of the hospital that manage infected patients, such as operating rooms, outpatient departments (OPDs), or in-patient wards (IPWs).
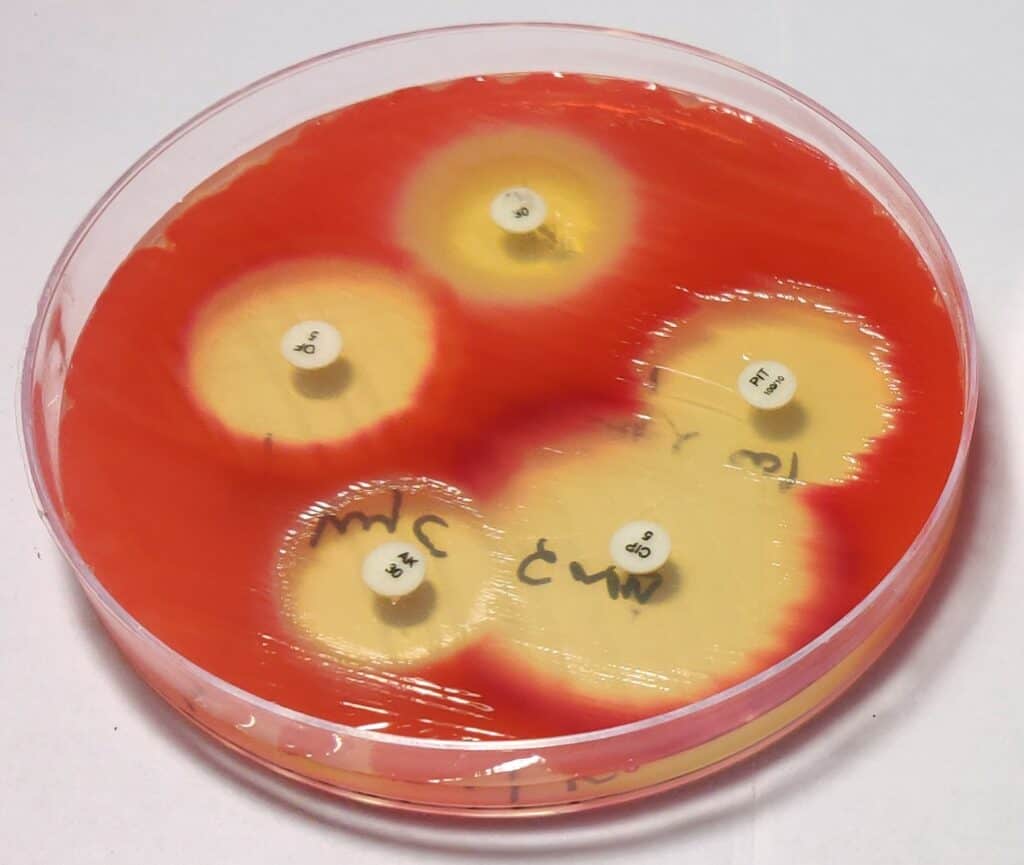
Bacterial pathogen with increasing resistance, PC: Sanchita Mitra, LVPEI
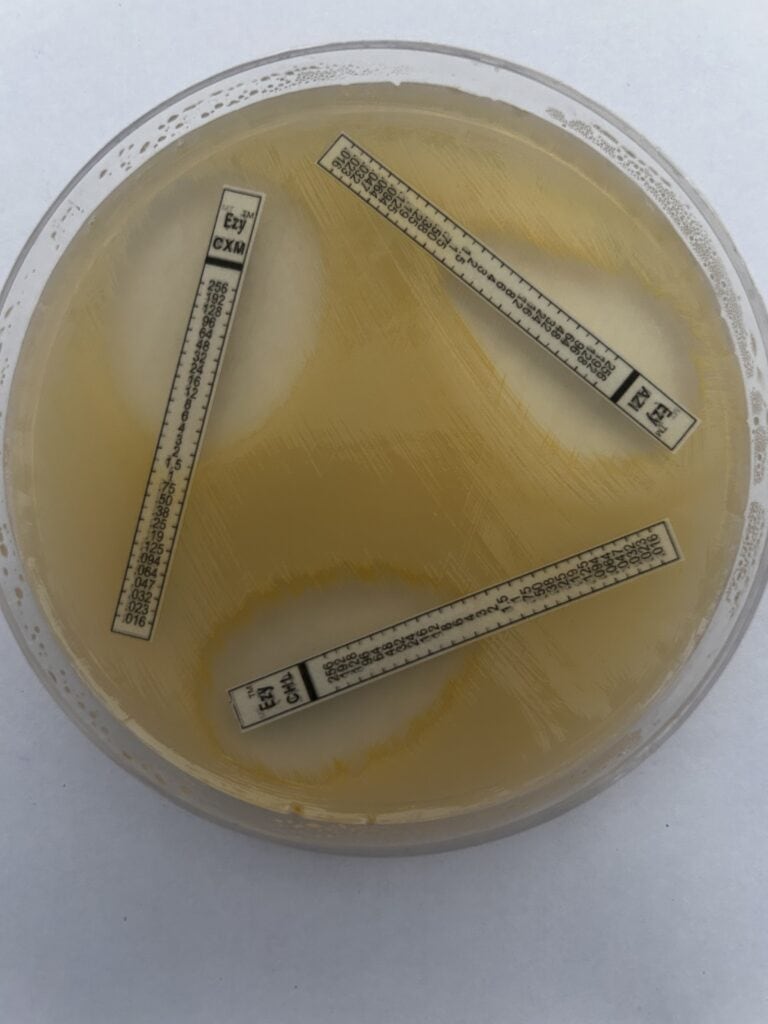
Bacterial pathogen resistant to multiple antibacterials on lab testing, PC: JMC, LVPEI
Antimicrobial resistance is a fast-changing field. To cope with these changes, hospitals use microbiology lab data of the previous year to build antibiotic susceptibility profile for all the major pathogens isolated from hospital infections. This data is used to build the stewardship program for the next year. The program policy document formulates the preferred antibiotic for each condition. In the case of ocular infections, for example, antibiotics used, and their mode of delivery, changes depending on where the infection has taken root: the surface of the eye, or its inner regions.
Every year, the susceptibility profiles are re-checked against data from the hospital, and updated based on any change in resistance to the specific antibiotics used. Antibiotics that kill less than 90% of sample bacteria are the first-line antibiotics, and those that kill over 90% of the sample are second-line drugs in the policy. Most higher order antibiotics, including the last-line reserve drugs like Colistin are second-line antibiotics.
When a doctor detects a specific infection in their clinic, the stewardship policy mandates that they prescribe the first-line antibiotic stipulated in the stewardship policy first. If there is no response, or if the infection is severe, only then are they allowed to escalate to the second-line antibiotics—this use is treated as an emergency. This approach preserves the potency of the second-line drugs against drug-resistant strains of that infection.
A good hospital uses such stewardship programs to preserve pathogen susceptibility to few last-line antimicrobials thereby limiting the spread of AMR. It is also important that hospitals audit compliance to the policy regularly.
Infection Control and Waste Management
Atul Gawande, the famed American surgeon and writer notes that, “we are used to thinking of doctoring as a solitary, intellectual task. But making medicine go right is less often like making a difficult diagnosis than like making sure everyone washes their hands.” The basics of infection control play a key role in ensuring that hospital-borne infections do not spread. Apart from hand hygiene, hospitals need to ensure that staff are properly vaccinated, the hospital material like clothing and bed linen are appropriately handled, and all potential surfaces are thoroughly and systematically cleaned. It is always the basics that will save us, and paradoxically, are the hardest to implement. Therefore, timely education and training of hospital staff on protocols and processes is key.
Hospitals also end up disposing antibiotics and other antimicrobial medication in relatively large quantities. Hospital sewage is a major source of AMR emergence in the environment. So it is critical that partially-used strips of antibiotics, or expired/near-expired medicine are not disposed in to the hospital sewage lines. Such expired or discarded medicines are sent to a biomedical waste treatment plant, where the waste is either incinerated or treated using waste autoclave methods. The mode of disposal is based on applicable guidelines and in coordination with the respective State and Central Pollution Control Boards.
The Social Contract
Hospitals, though vital, are only a small part of our efforts to tackle AMR. As a society, we are over-exposed to antibiotics everywhere: in medicated soaps and floor cleaners, in over-the-counter pills and syrups, and in the disposal of all these drug-laced products into our sewage and water. A far bigger source is the unregulated use of antimicrobials in agriculture, pisciculture, and the dairy industry. Their wholesale use has been pushing extensive drug resistance in the environment.
Unless all stakeholders–the doctors, scientists, patients and their families, and the market–work together, we will not be able to turn the tide on antimicrobial resistance, ending our decades-long success against infections.
A version of this piece previously appeared here.
Cover image: Representative photo for a hospital setting. Photo Credit: LV Prasad Eye Institute
—
About the Author: Dr. Sanchita Mitra is a Consultant Microbiologist at the LV Prasad Eye Institute (LVPEI), Hyderabad. She plays a key role in the institute’s AMR Stewardship Team and serves as the Convener of the Hospital Infection Control Committee (HICC). In these capacities, she is actively engaged in formulating protocols and guiding decisions on ocular infection control practices. Her primary research focuses on AMR, with a particular emphasis on both antibacterial and antifungal resistance, and she is dedicated to advancing solutions to address the growing AMR challenge.